Helium hydride ion (HeH+) was the first molecule that formed when, almost 14 billion years ago, falling temperatures in the young universe allowed recombination of the light elements produced in the Big Bang.
Scientists have detected the most ancient type of molecule in our universe in space for the first time ever.
Helium hydride ion (HeH+) was the first molecule that formed when, almost 14 billion years ago, falling temperatures in the young universe allowed recombination of the light elements produced in the Big Bang.
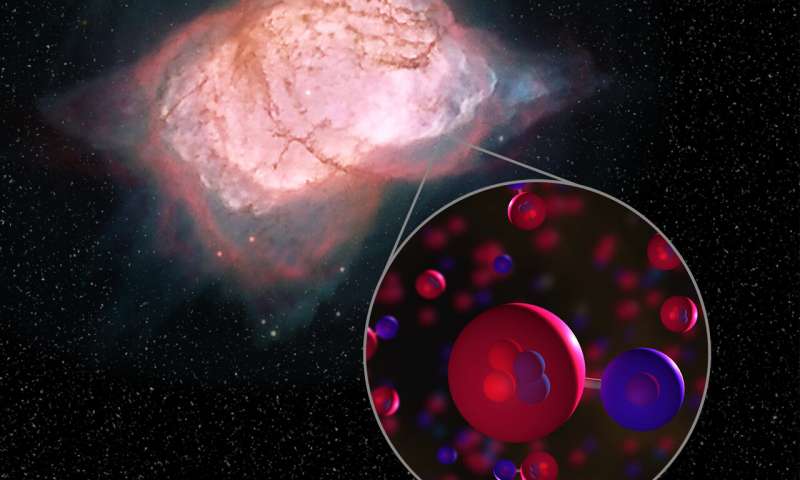
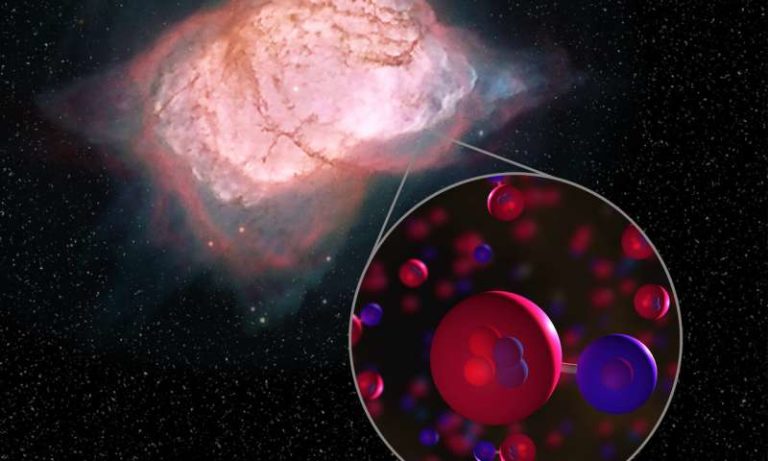
Operating the GREAT far-infrared spectrometer onboard the flying observatory SOFIA, an international team reported unambiguous detection of the molecule towards the planetary nebula NGC 7027.
During the dawn of chemistry when the temperature in the young universe had fallen below 4000 Kelvin, the ions of the light elements (hydrogen, helium, deuterium and traces of lithium) produced in Big Bang nucleosynthesis recombined in reverse order of their ionisation potential.
At that time, ionised hydrogen and neutral helium atoms reacted to form HeH+, said researchers from The Max Planck Institute for Radio Astronomy (MPIfR) in Germany.
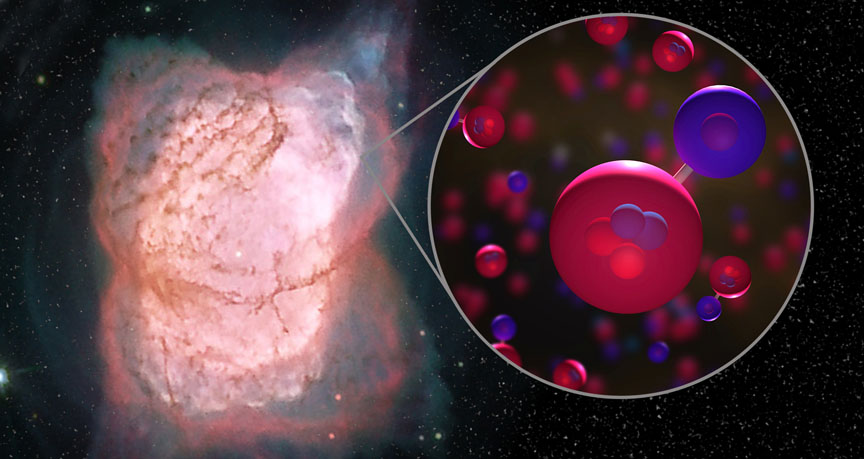
Helium combined first with free electrons to form the first ever neutral atom, according to the study published in the journal Nature.
At that time hydrogen was still ionised or present in form of bare protons.
Despite its importance in the history of the early Universe, HeH+ has so far escaped detection in astrophysical nebulae — cloud of gas and dust in outer space.

Helium atoms combined with these protons into the helium hydride ion HeH+, the universe’s first molecular bond.
As recombination progressed, HeH+ reacted with then neutral hydrogen and created a first path to the formation of molecular hydrogen — marking the beginning of the modern universe.

[zombify_post]













0 Comments